.
CMC, MSAT, CQV Consulting:
-
Scale-Up & Tech Transfer: Risk assessments, Process Gap Assessments, and MPR generation
-
Process Characterization & Validation: PVMP, PPQ Protocols & Reports, and CPV execution
-
URS, FAT/SAT, IQ/OQ/PQ documents
What We Do ( OUR SOLUTION)
End-to-end Phase appropriate CMC support
We specialize in:
-
Tech Transfer Packages & Protocols
-
Process Characterization (DOE-driven)
-
Process Validation (PPQ) Strategy & Documentation
-
Continued Process Verification (CPV) Frameworks
-
Annual Product Quality Review (APQR) Documents
-
Risk Assessments (FMEA, Control Strategy, Design Space).
-
Equipment CQV documents
-
Modalities: Oligonucleotide, mAb, AAV & Viral Vector CMC documentation
Every document is structured, compliant, and scientifically justified.
WHY BENCH2BATCH™
Bench2Batch is led by Naveenganesh Muralidharan, a 20-year biopharmaceutical MSAT expert who has:
-
Supported programs from development → launch
-
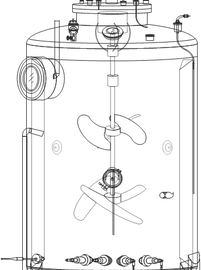
Designed scale-up models from 250 mL → 20,000 L bioreactors
-
Created online scale-down models, shear assessments, CFD simulations
-
Built DOE-based characterization protocols for biologics & oligonucleotides
-
Authored 25+ publications in Bioprocess International and American Pharmaceutical Review on Biopharma CMC activities
-
Led successful tech transfers across biotechnology platforms
-
Developed digital tools using Python, JavaScript, Retool, and advanced analytics
-
You get more than documentation — you get a true technical partner.

On-Line Bioreactor Scale-Up & Scale Down Tools
These online bioreactor calculators simplify the process of scaling up or down between bench and production scale. They offer tools to predict pCO2, assess shear from impellers and sparging, and
calculate OTR and KLa. This helps users optimize process conditions and improve scalability
across different reactor sizes.
Welcome to Bench2batch! We're thrilled to have you here.
On-line Engineering and Applied statistics tools in Process Validation
Comprehensive biopharma process validation suite featuring microbial limits, endotoxin modeling, mixing risk, PPQ run justification, OC curve sampling, and CI/TI/Ppk acceptance tools.
Note: These online applications represent limited-scope demo versions. Bench2Batch delivers deeper, fully customized, and regulatory-aligned process validation solutions. Contact us to learn more

Technical Insights